Interview with Eero Mäki-Lohiluoma, Quality Assurance Manager and Qualified Person at Orion Corporation
Scientific mindset and approach to problem solving is one of the key success factors at Top Data Science and in our customer projects. In addition to AI and software solutions, sometimes this approach leads to scientific publications and research papers with our customers and partners.
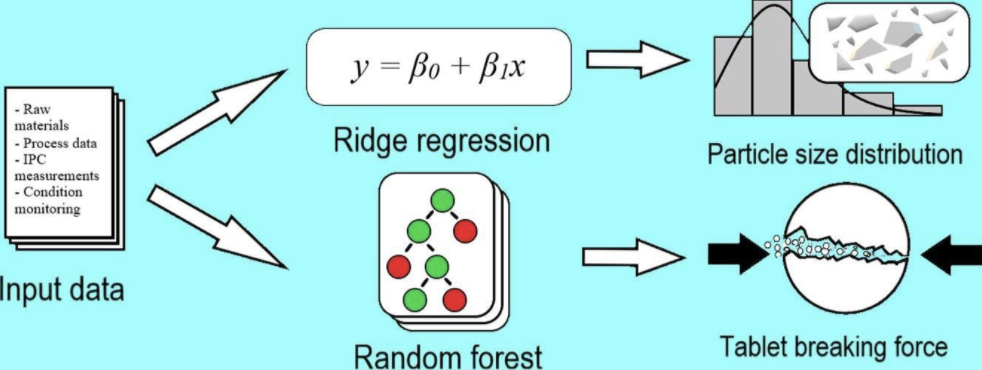
In our recent co-creation project with Orion Pharma, we discovered how to use Machine Learning to extract and utilize relevant product quality information in Pharma industry manufacturing.
In this blog post Timo Heikkinen, former CEO and Co-Founder at Top Data Science, is interviewing Eero Mäki-Lohiluoma, Quality Assurance Manager and Qualified Person, who is one of the project leaders at Orion Pharma.

As part of his Industrial Pharmacy Specialisation Studies, Eero was the main author for the publication called: ‘Use of machine learning in prediction of granule particle size distribution and tablet tensile strength in commercial pharmaceutical manufacturing’. This novel publication will be released on ‘International Journal of Pharmaceutics’, Volume 609, 20 November 2021, 121146
https://www.sciencedirect.com/science/article/abs/pii/S0378517321009522?dgcid=coauthor
Could you tell a bit about yourself, your professional and academic background?
I’m M. Sc. (Pharm.) graduated from University of Helsinki 2015. I started at Orion in December 2014 as a part time QA pharmacist. In September 2015 I transferred to a pharmaceutical expert role in Orion own tablet production. That was a start for many great years. During the 4 and a half years, I performed maintenance tasks for tablet and injection products, I was part of the continuous manufacturing exploration project and developed commercial scale manufacturing processes for tablet products. Most of all, I met wonderful and skilled people from whom I was able to learn a lot.
You are working in the pharma industry in quality assurance, but also do research as a pharmacist?
I started at Orion QA as a QP in March 2020, which is also my current position. In 2017, when working as a pharmaceutical expert, I decided to apply to the Industrial Pharmacy Specialisation program. Scientific publication was part of the studies. I graduated from the program in June 2021.
As a pharmaceutical expert, I was fortunate to work with people with different backgrounds. In this multitalented group, I found myself asking the same questions: how to utilize data for better decisions making and for developing robust manufacturing processes. This eventually gave me the opportunity to work with Top Data Science’s data scientists and write a publication of our studies.
How would you describe the quality assurance work and GMP in the pharmaceutical industry?
Quality assurance is the core of the pharmaceutical industry. Everything is based on Good Manufacturing Practices, GMP. The concept of GMP has been formed from a need to protect the end-users and to create a reliable drug manufacturing system. The history is well known from unfortunate crises when something has gone wrong. Ultimately, the idea of GMP is to manufacture drugs in a way that the risk of human error is mitigated as much as possible and that the safety and effectiveness of drugs is ensured. I think it is a smart and justified way. QA is in a key role to enable, endorse and require sufficient GMP.
In Finland the requirement for GMP is set in the Lääkelaki-law (hyvät tuotantotavat). Furthermore, there are many more guidelines that are expected to be followed in good manufacturing practices, such ICH guidelines, Pharmacopoeias, EMA and national CA guidelines, IPSE guidances, ISO standards etc.
How do you see the Quality by Design (QbD) concept compared to the Quality by Testing (QbT) approach?
The QbD mindset is about understanding and controlling the manufacturing process in a way that the risk of an unexpected incident is mitigated. Doesn’t this sound like a good fit in a GMP environment? This being said, the QbD product development is resource-consuming and requires skilled experts. However, I believe that the time spent on QbD product development will pay off, in multiple different ways. In the worst case, life with a QbT product can be compared to a walk through a lake with thin ice. You might get lucky and find a way without getting wet, but it can also be a hell of a struggle.
Do you think Machine Learning, AI and data driven analytics will create tangible results and benefits in your work and in pharma manufacturing?

Absolutely. I don’t think there is any other way to understand your process than looking at the data. Most of the time the data requires further analysis, and only suitable algorithms can give you the answer that you are looking for.
In the big picture, there is a slow transformation process from doing things that feel right and seem to be working to a mindset where the decisions are expected to be done based on data and science. This is not always controversial, but it is important to be able to look deeper into the reasons and justifications for the common ideas and practices. Data science offers us a great opportunity to learn and understand different phenomena, and ultimately help us to grow as a person. I believe that the ones who understand this will be the winners in the future, and this is not only related to pharma manufacturing.
Are there some challenges or obstacles in using AI and data in large companies and in the pharma industry?
Like in all transformations, there are challenges to make the next steps. Especially the first ones. From my experience, in many pharma companies, data is still only on paper and collecting dust in the paper archives. If electronic systems are used, there are multiple different software and data formats, which causes a lot of work for data analytics before the data is in an usable format. The reasons behind the paper-based documentation and complex data architectures are understandable, but it is important to work cross-functionally to overcome these challenges. The needs of end-users should be one of the top priorities.
It is also important to measure and collect the data from relevant things. Without relevant factors in the input data, the AI cannot predict the outcome reliably. The fit of AI to the GMP environment also raises puzzling questions, since the outcomes of the models are usually not self-evident. However, I believe that there are solutions for these questions. At times, people say that GMP is restricting innovation. This should not be the case.
How did the collaboration with Top Data Science start?
Eero Kiljunen introduced the company first to Ari Kauppinen and then I jumped in. We had a Proof-of-Concept project in summer of 2020 where one of our first targets was to predict tableting parameters with machine learning models that would allow tableting of compliant tablets from the very beginning of the tableting process. This would reduce loss in process ramp up and improve yield. Unfortunately, we did not achieve this target due to reasons related to the input data. We switched the scope to predict the particle size distribution and tablet tensile strength, which was also the subject of the scientific publication.
How would you describe working with a specialist AI and Machine Learning company, like Top Data Science?
It was exciting and beneficial. Top Data Science has had similar projects in other fields of business, which enabled them to have a quick start with the actual work. The flexibility of the team was also highly appreciated when tackling the challenges throughout the project.
How do you see a co-creation type of working approach, that combines your in depth process and pharmaceutical knowledge and AI & software expertise?
It is a must. I am in the impression that there are quite a few people who understand the process challenges as well as masters the data analytics. Both require constant learning to stay on top of things. It is also not meaningful to try to tackle the problems by yourself. In the end, we all are in front of the same challenges, which can be solved in a sustainable way only through collaboration.
–
About Orion Corporation:
Orion Corporation, founded in 1917 and headquartered at Espoo, Finland, is a globally operating Finnish company which develops, manufactures and markets human and veterinary pharmaceuticals and active pharmaceutical ingredients for global markets.
Revenue: 1.078 billion EUR (2020)
Headquarters: Espoo, Finland
Number of employees: 3,300 (2020)
https://www.orion.fi/




